It wasn’t in Altamira or France: the oldest cave painting on the planet (67,800 years old) appears in a remote cave in Asia and forever changes the map of human creativity
For much of the last century, the story of cave art has centered on Europe, from the painted halls of Lascaux Cave to the…..
It’s not a virus, it’s not a bacterium… it’s something stranger: Japanese scientists discover a life form that doesn’t fit into any known biological category
Inside what looks like an ordinary drop of seawater, researchers have found something that almost slips out of biology’s rulebook. A Japanese led team,…..

The Cybertruck already has a historic nickname: “the new Ford Edsel,” and when you’re compared to the biggest commercial failure of the 20th century, things are going very, very badly

They measure between 2 and 6 centimeters, but their skin can hide one of the most lethal toxins on the planet, which is why the “poison dart frog” is so fearsome
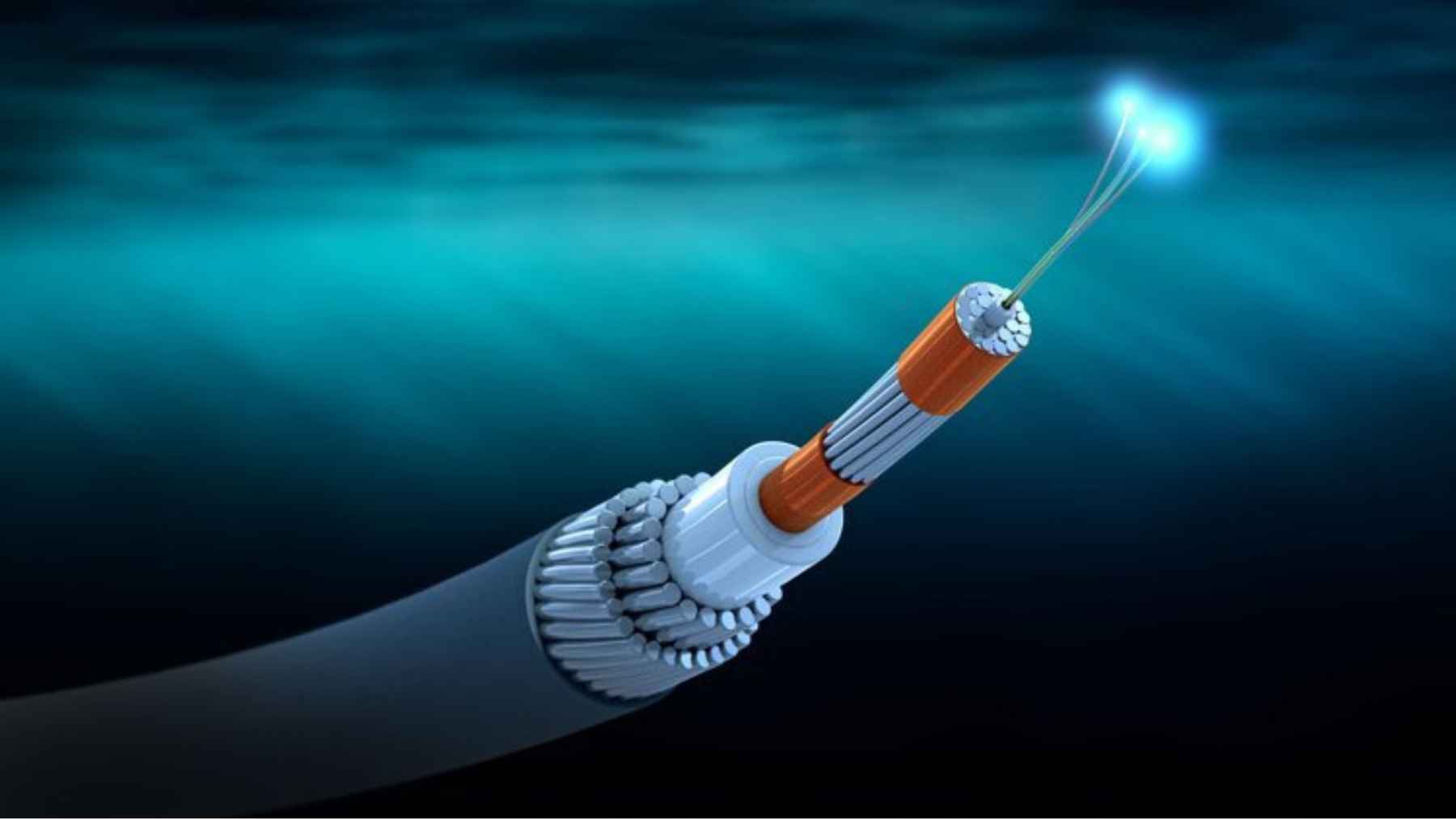
The cables that carry 99% of the internet could become a giant microphone for detecting earthquakes, tsunamis and even whales

Mexico pulls a “land-based Panama Canal” out of its hat: 303 km across the Isthmus of Tehuantepec to connect the Pacific and the Gulf without passing through locks

China has just launched a hydroelectric “beast” in Tibet that generates 11 billion kWh per year, and whose dam, at 295 meters high, is almost as tall as the Eiffel Tower

It was buried under 30 meters of polar ice and had been missing since 1967: NASA unexpectedly finds an old secret nuclear test site in the middle of the Arctic
Science
It wasn’t in Altamira or France: the oldest cave painting on the planet (67,800 years old) appears in a remote cave in Asia and forever changes the map of human creativity
For much of the last century, the story of cave art has centered on Europe,…..
It’s not a virus, it’s not a bacterium… it’s something stranger: Japanese scientists discover a life form that doesn’t fit into any known biological category
Inside what looks like an ordinary drop of seawater, researchers have found something that almost…..
It was buried under 30 meters of polar ice and had been missing since 1967: NASA unexpectedly finds an old secret nuclear test site in the middle of the Arctic
When a NASA research jet flew over northern Greenland in April 2024, scientists expected to…..
It wasn’t just a simple stroll through the galaxy: 14 million years ago, the solar system passed through a colossal wave of gas and stars that may have triggered the great Miocene glaciation
About 14 million years ago, our solar system sailed through a vast ribbon of gas…..
An unprecedented view of the Sun is about to arrive: the Parker probe passed through the solar atmosphere at approximately 429,000 miles per hour (mph) and recorded data that could solve one of science’s oldest mysteries
What happens when a spacecraft skims the atmosphere of our star, in a region hotter…..
India joins the most exclusive club in space: it has achieved its first docking between satellites in orbit and is already dreaming of having its own space station by 2035
On January 16, 2025, India became the fourth nation to complete a successful uncrewed space…..
NASA and China warn that the Moon could be hit by a 60-meter rock and that the impact could trigger a meteor storm that would knock out the Internet, satellites, and GPS for years
What if the most spectacular sky show of the century came with a very down-to-Earth…..
The day when dozens of sea turtles panicked and fled at full speed under the sea 79 million years ago: Italian climbers have just found their perfectly preserved footprints after an avalanche caused by a prehistoric earthquake
Rock climbers scaling a limestone cliff on Monte Conero on Italy’s Adriatic coast noticed something…..
Excavating beneath an Italian square, they finally uncover Vitruvius’ lost building: the basilica described in 19 BC comes to life in the 21st century
After more than five hundred years of searching, archaeologists in the coastal city of Fano…..
China is building a giant hypergravity centrifuge capable of accelerating processes that take centuries to complete in nature
What would you do with a machine that makes gravity nearly two thousand times stronger…..
Mobility

The Cybertruck already has a historic nickname: “the new Ford Edsel,” and when you’re compared to the biggest commercial failure of the 20th century, things are going very, very badly
In 2025, the stainless steel Cybertruck went from viral star to cautionary tale. Instead of the quarter…..

China promises the “holy grail” of electric vehicles in 2026: an Exeed with a solid-state battery that would exceed 1,500 km and, according to the company, withstand temperatures as low as -30 °C
Most electric car drivers still plan long trips around charging stops and worry about how far the…..
Goodbye to BMW, Mercedes, and Volkswagen: an economist warns that these brands could disappear before 2030
Energy
Economy
China has just found a 1,444-ton gold “monster” valued at more than $150 billion, and the strangest thing is that they say it is the largest find since 1949
China has confirmed the largest gold discovery in its modern history in the northeastern province…..
China processes around 90% of the world’s rare earths, but Sweden has just pulled an “ace up its sleeve” with 2.2 million tons of oxides in Per Geijer
High above the Arctic Circle, a long-running iron mine in northern Sweden has quietly revealed…..
Millions of people could lose their food this month following the implementation of a controversial law requiring 80 hours of work to retain SNAP benefits
New work rules for the Supplemental Nutrition Assistance Program are starting to hit home in…..
A list reveals that three countries are among the most indebted in the world in 2025, with figures exceeding 120% of GDP
Public debt rarely makes front page news compared with inflation or jobs, yet it quietly…..
The longest tunnel in Latin America took more than 10 years to build and now connects two key regions that were previously isolated by mountains
High in the central Andes, a new piece of concrete and steel is changing how…..
The United States and Japan want to spend $550 billion on synthetic diamonds, which says a lot about the problem with China
For most people, diamonds mean engagement rings and glittering store windows, not chip factories and…..
An iconic Chicago candy factory with nearly 100 years of history has filed for bankruptcy and could close permanently after losing millions
One of Chicago’s oldest candy makers has landed in bankruptcy court. Primrose Candy Company, a…..
China directly threatens Panama for canceling historic port contracts signed in the 1990s and warns that it will pay a high political and economic price if it does not rectify the situation
At the center of the latest clash between China and Panama sits the Panama Canal,…..
A renowned airline just accidentally discovered that it had a Boeing plane abandoned at an airport since 2012 and no one at the company had noticed for over a decade
We all misplace our keys or forget an old subscription. But an entire jetliner slipping…..
All U.S. Social Security numbers may need to be changed following a massive breach that is already being investigated as a national threat
Every American who has ever had a Social Security number may now be living with…..
Technology

This isn’t about hypersonic missiles: the USS Gerald R. Ford (CVN-78) has a serious operational problem, and it’s purely plumbing-related

Ukraine wants to move from being a “human shield” to a shield that thinks for itself: in the next six months, it will deploy an artificial intelligence-based air defense system capable of predicting Russian attacks and launching autonomous interceptors before you can blink

The “Internet of the future” has a very literal dark side: nearly 2,000 observations reveal that Amazon’s satellites shine brighter than promised
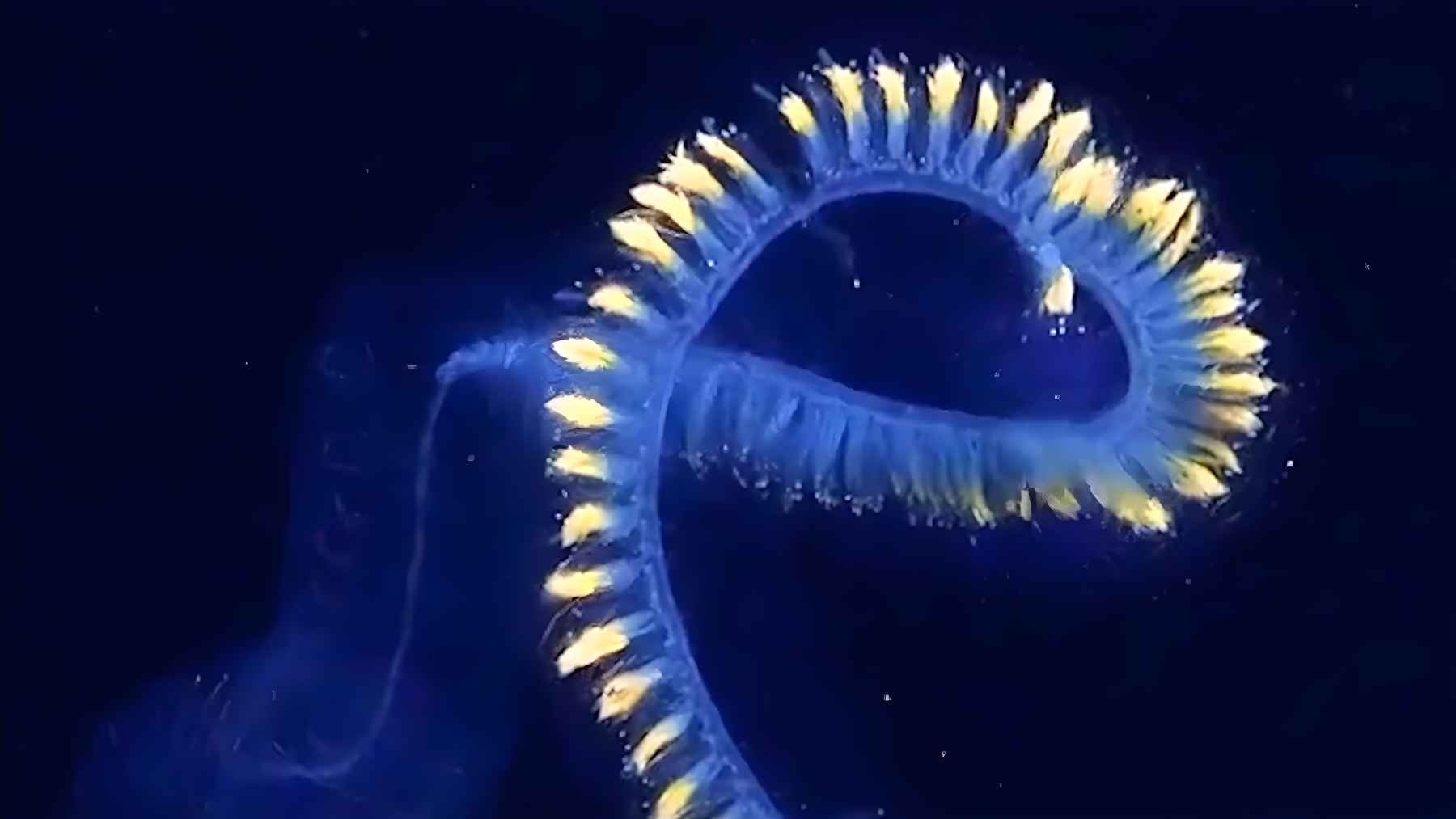
They lowered a robot to 6,000 meters, and what appeared on the camera looked like something from another planet: a 15-meter translucent “creature” floating in total darkness

A Chinese humanoid robot has just made history at -47.4 °C, taking more than 130,000 steps on the ice in Xinjiang and even “drawing” an Olympic emblem

A bottle-sized “windmill” that promises to save your cell phone in the middle of nowhere, Shine 2.0 says it charges with just 8 mph of wind and weighs 3 pounds
Environment
They measure between 2 and 6 centimeters, but their skin can hide one of the most lethal toxins on the planet, which is why the “poison dart frog” is so fearsome
If you have ever pressed your face against the glass of a zoo terrarium to…..
Mexico pulls a “land-based Panama Canal” out of its hat: 303 km across the Isthmus of Tehuantepec to connect the Pacific and the Gulf without passing through locks
In southern Mexico, bulldozers and rail crews are reshaping one of the narrowest slices of…..
A European satellite confirms what many doubted: between 2019 and 2023, California added hundreds of zero-emission vehicles per area, and the TROPOMI satellite detected a measurable decrease in pollution from space
If you have ever inched through city traffic and watched exhaust hang over the road,…..
Goodbye to mining in the heart of the jungle: Colombia makes history by declaring its entire Amazon region free of hydrocarbons and mega-mining
In a decision that could reshape the future of the rainforest, Colombia has moved to…..
In 2019, China did something that seems crazy: “cover” glaciers with giant blankets. Now, in 2026, data shows how much they actually slow down melting
Back in 2019, a group of researchers from the Chinese Academy of Sciences hiked up…..
They look like simple puddles of water, but every time it rains, they activate a prehistoric ecosystem whose lineage began more than 100 million years ago and which the European Union considers a priority
After weeks of heavy rain, the open plains around São Marcos da Ataboeira in the…..
At a depth of 3.2 km and next to a volcano, they have recorded the largest octopus nursery on the planet, the size of 233 soccer fields and full of incubating mothers
Two miles beneath the Pacific Ocean near an extinct volcano off California, the seafloor turns…..
They tried to slow down the advance of the Sahara with millions of bees… and they “melted” at over 70 °C. However, the solution that works is not biology, but geometry on the ground
In one of the harshest corners of the planet, the Sahara Desert has quietly put…..
Trending

The United States issues an ultimatum that could change the map of North American airspace: if Canada renounces the purchase of 88 F-35 fighter jets, Washington says it will have to “take command” of Canadian airspace and rethink the entire NORAD in 2026

The United States grew tired of the northern lights “blinding” its radars and in 2026 launched FROSTY, a DARPA plan to turn that Arctic noise into its new surveillance weapon

A Geran-5 is shot down, and what Ukraine finds inside looks like a kind of military globalization: a Chinese TELEFLY turbojet engine, carbon fiber, and foreign chips in a drone carrying a 90 kg payload

“They’re not ships, they’re floating brains”: why the Spanish Navy is redesigning an entire naval base so that the F-110s, valued at more than 4.3 billion, can operate without limits from 2026 onwards

Saudi Arabia’s “impossible cube” no longer exists. On January 28, 2026, they stopped the Mukaab, a 400-square-meter colossus that would have swallowed up 20 Empire State Buildings